Palladium-catalyzed remote C–H functionalization of 2-aminopyrimidines†
Abstract
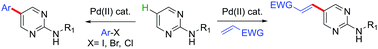
A straightforward strategy was developed for the arylation and olefination at the C5-position of the N-(alkyl)pyrimidin-2-amine core with readily available aryl halides and alkenes, respectively. This approach was highly regioselective, and the transformation was achieved based on two different (Pd(II)/Pd(IV)) and (Pd(0)/Pd(II)) catalytic cycles.


 Please wait while we load your content...
Please wait while we load your content...