Palladium-catalyzed diastereo- and enantioselective allylic alkylation of oxazolones with 1,3-dienes under base-free conditions†
Abstract
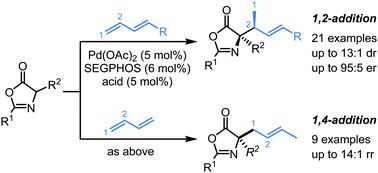
Herein, we report a highly diastereo- and enantioselective allylic alkylation of oxazolones with 1,3-dienes by palladium-hydride catalyst under base-free conditions. With DTBM-SEGPHOS as the chiral ligand, a series of enantioenriched oxazolones bearing tertiary carbon centers were synthesized from substituted 1,3-dienes via exclusive 1,2-addition with moderate to good diastereoselectivities and high enantioselectivities. When simple 1,3-butadiene was used as the allyl precursor under this base-free catalytic system, 1,4-addition products were obtained in good yields with high regioselectivities.


 Please wait while we load your content...
Please wait while we load your content...