Computational and experimental identification of strong synergy of the Fe/ZnO catalyst in promoting acetic acid synthesis from CH4 and CO2†
Abstract
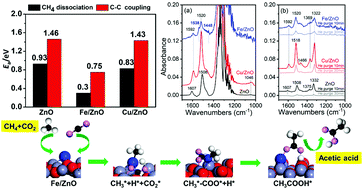
DFT calculations have identified reaction pathways for acetic acid synthesis from CO2 and CH4 on ZnO, Cu/ZnO and Fe/ZnO surfaces. Fe/ZnO exhibits strong synergy in facilitating CH4 activation, dissociation and C–C coupling. Thus, the surface acetate formation is significantly enhanced. The DFT predictions have been confirmed by in situ DRIFTS experiments.


 Please wait while we load your content...
Please wait while we load your content...