Asymmetric dearomatization of 2-nitrobenzofurans by organocatalyzed one-step Michael addition to access 3,3′-disubstituted oxindoles†
Abstract
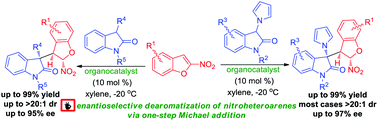
An efficient enantioselective dearomatization of 2-nitrobenzofurans was realized via an organocatalyzed one-step Michael addition process. This method provides a facile strategy to access a range of structurally diverse 3,3′-disubstituted oxindoles, which feature an intriguing combination of two privileged motifs including 3-pyrrolyl-substituted-oxindoles and 2,3-dihydrobenzofurans substructures, in excellent results.


 Please wait while we load your content...
Please wait while we load your content...