One-pot oxidative hydrolysis-oxidative cleavage of 7-borylindoles enables access to o-amidophenols and 4-acylbenzoxazoles†
Abstract
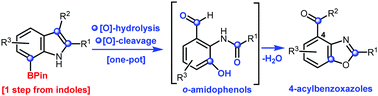
7-Borylindoles undergo a one-pot oxidative-hydrolysis of the arylboronate and oxidative cleavage of the indole C2–C3 double bond to afford o-amidophenol derivatives. Subsequent cyclisation delivers benzoxazoles bearing an acyl group at C4, a substitution pattern common to fungal-derived benzoxazole alkaloids. Using 7-borylindoles as substrates to access functionalised o-amidophenols circumvents the difficult preparation of these compounds from arenes, streamlining access to substituted 4-acylbenzoxazoles in the process.


 Please wait while we load your content...
Please wait while we load your content...