The crystal structure of a natural DNA polymerase complexed with mirror DNA†
Abstract
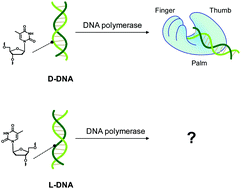
The intrinsic L-DNA binding properties of a natural DNA polymerase was discovered. The binding affinity of Dpo4 polymerase for L-DNA was comparable to that for D-DNA. The crystal structure of Dpo4/L-DNA complex revealed a dimer formed by the little finger domain that provides a binding site for L-DNA.


 Please wait while we load your content...
Please wait while we load your content...