Structure of micelle bound cationic peptides by NMR spectroscopy using a lanthanide shift reagent†
Abstract
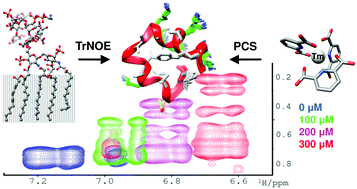
[Tm(DPA)3]3− was used to generate multiple, paramagnetic nuclear Overhauser effect NMR spectra of cationic peptides when weakly bound to a lipopolysaccharide micelle. Increased spectral resolution combined with a marked increase in the number of distance restraints yielded high resolution structures of polymyxin and MSI-594 in the liposaccharide bound state.


 Please wait while we load your content...
Please wait while we load your content...