Highly enantioselective electrosynthesis of C2-quaternary indolin-3-ones†
Abstract
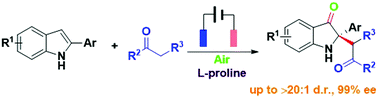
A highly enantioselective and direct synthesis of C2-quaternary indolin-3-ones from 2-arylindoles by combining electrochemistry and organocatalysis is described. Excellent enantioselectivities (up to 99% ee) and diastereoselectivities (>20 : 1) can be obtained through anodic oxidation in combination with asymmetric proline-catalyzed alkylation in an undivided cell under constant-current conditions.


 Please wait while we load your content...
Please wait while we load your content...