Dicarbonylative benzannulation of 3-acetoxy-1,4-enynes with CO and silylboranes by Pd and Cu cooperative catalysis: one-step access to 3-hydroxyarylacylsilanes†
Abstract
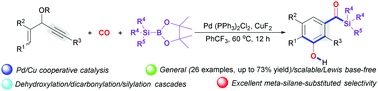
A new, general Pd/Cu-cocatalysed dicarbonylative benzannulation of 3-acetoxy-1,4-enynes with CO and silylboranes is described. The method utilizes CO as both a one-carbon (C1) unit and an external addition functional reagent to achieve an unprecedented dicarbonylative benzannulation process, and represents a facile, efficient route to 3-hydroxyarylacylsilanes. Mechanistically, the silyl-Cu intermediate formed from CuF2 and silylboranes, and silyl-Pd intermediate generated by transmetallation are two key factors for successfully targeting the reaction and selectivity.


 Please wait while we load your content...
Please wait while we load your content...