A rhodium(ii) catalysed domino synthesis of azepino fused diindoles from isatin tethered N-sulfonyl-1,2,3-triazoles and indoles†
Abstract
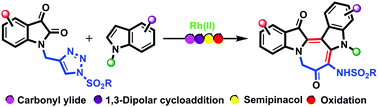
An efficient and convenient protocol for the synthesis of a novel class of azepino fused diindoles from isatin tethered N-sulfonyl-1,2,3-triazoles and indoles has been disclosed. The reaction proceeds via denitrogenative aza-vinyl rhodium carbene formation to give a carbonyl ylide, which with indole results in 1,3-dipolar cycloaddition followed by sequential semipinacol rearrangement/ring expansion/oxidation to produce azepino fused diindoles. The reaction shows a broad substrate scope giving up to 81% yield. Furthermore, reversible catalytic hydrogenation and photophysical studies were carried out to demonstrate the application of these molecules.


 Please wait while we load your content...
Please wait while we load your content...