Ligand-activated BRET9 imaging for measuring protein–protein interactions in living mice†
Abstract
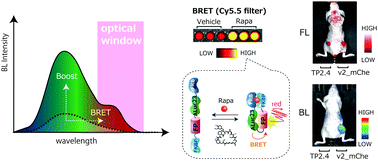
Bioluminescence resonance energy transfer (BRET) is a commonly used assay system for studying protein–protein interactions and protein folding in vivo. Conventional BRET systems have solely depended on an overlap of the energy donor and acceptor spectra. In this study, we engineered a conceptually unique ligand-activatable BRET system (termed BRET9), where a full-length Artificial Luciferase variant 23 (ALuc23), acting as the energy donor, is sandwiched between a protein pair of interest, FRB and FKBP12, and linked to a fluorescent protein as the energy acceptor. A specific ligand, rapamycin, then activates inter- and intramolecular interactions of FRB and FKBP12, which develop molecular strain in the sandwiched ALuc23 to accelerate further folding. We found that this system greatly enhanced both the total bioluminescence spectrum and the BRET signal in the far-red (FR) region. We characterized the molecular construct by studying 18 different designs categorized into four groups. The best BRET system design allowed an approximately 5-fold enhancement of the bioluminescence intensities in the FR region. This new BRET system provides a robust ligand-activatable platform that efficiently reports FR bioluminescence signals in cells and living animal models.


 Please wait while we load your content...
Please wait while we load your content...