Chemical synthesis of a haemathrin sulfoprotein library reveals enhanced thrombin inhibition following tyrosine sulfation†
Abstract
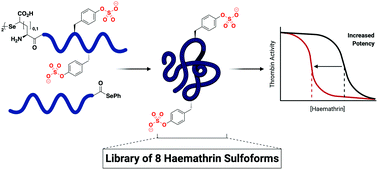
The haemathrins are tick-derived thrombin-inhibiting proteins predicted to be post-translationally sulfated. This study reports the ligation-based assembly of eight homogeneously sulfated variants of haemathrin-1 and haemathrin-2. Functional assays revealed a two orders-of-magnitude enhancement in thrombin-inhibitory potency by tyrosine sulfation, thus reinforcing the crucial role of this post-translational modification for the activity of anticoagulant proteins.
- This article is part of the themed collection: The Chemical Biology of Peptides


 Please wait while we load your content...
Please wait while we load your content...