Canola oil extraction in conjunction with a plastic free separation unit optimises microplastics monitoring in water and sediment
Abstract
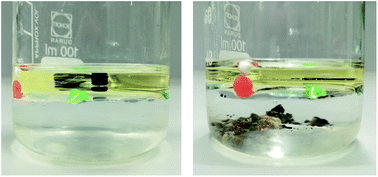
Microplastics are widely distributed in the environment and to define contamination hot spots, environmental samples have to be analysed by means of cost-as well as time-efficient and reliable standardised protocols. Due to the lipophilic characteristics of plastics, oil extraction as a fast and density-independent separation process is beneficial for the crucial extraction step. It was extensively validated (480 experiments) in two test setups by using canola oil and a cost-effective, plastic-free separation unit with spiked microplastics (19 different polymer types) in the density range from ρ = 11–1760 kg m−3 and in the size range from 0.02–4.4 mm. Thus, an innovative, new method combination was developed and profoundly validated for water and sediment samples using only a short settling time of 15 minutes. Some experiments were also carried out with zinc chloride to obtain additional reference data (particles ≤ 359 μm). The total mean recovery rate was 89.3%, 91.7% within the larger microplastic fraction and 85.7% for the small fraction. Compared to zinc chloride (87.6%), recovery rates differed not significantly with oil (87.1%). Furthermore, size limits were set, since the method works best with particles 0.02 mm ≥ d ≤ 3 mm. The proposed method exhibits higher efficiency (84.8% for 20–63 μm) for the potentially most harmful microplastic size fraction than the classic setup using brine solution. As a result, oil is a comparably effective separation medium and offers further advantages for separating water and sediment samples due to its density independence, simple and fast application and environmental friendliness. Based on this, a new extraction protocol is presented here that confirms oil separation as a sound and effective separation process in microplastic analysis and identifies previously missing information.


 Please wait while we load your content...
Please wait while we load your content...