Screening and application of boron difluoride complexes of curcumin as colorimetric and ratiometric fluorescent probes for bisulfite†
Abstract
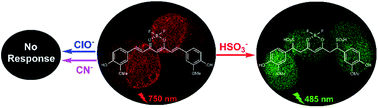
Bearing a unique D–π–A–π–D structure, curcumin (Cur) and its difluoroboron complex can be used as fluorophores to construct fluorescent probes with β-diketone or phenolic hydroxyl groups as the recognition sites. With α,β-unsaturated ketone as the potential Michael acceptor, Cur and its four derivatives were screened for their response to cyanide, hydrogen sulphide, hypochlorite and bisulfite in aqueous solutions. It was found that only difluoroboron curcumin (Cur-BF2) and difluoroboron dimethylated curcumin (Cur-Me-BF2) responded to bisulfite selectively along with color changes from blue or pink to colorless. Good linear relationships were observed between the fluorescence intensity ratio I485/I750 or I490/I610 and the concentration of bisulfite; then, the detection limits were determined to be 5.22 ppb and 12.48 ppb for Cur-BF2 and Cur-Me-BF2, respectively. The sensing mechanism was attributed to the addition of bisulfite to the difluoroboron-complexed α,β-unsaturated ketone to form disulfonic acid, which was confirmed by 1H NMR spectroscopy and HRMS. All the results suggested that Cur-BF2 and Cur-Me-BF2 could be used as colorimetric and ratiometric fluorescent probes for the sensitive, selective and quick-response detection of bisulfite. Moreover, the bioimaging of bisulfite in living H1975 cells with Cur-BF2 was performed successfully.


 Please wait while we load your content...
Please wait while we load your content...