In situ hemolysis in a three-dimensional paper-based device for quantification of intraerythrocytic analytes†
Abstract
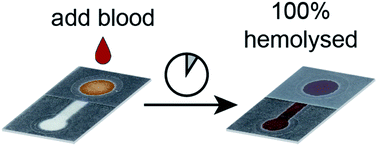
Blood-based diagnostics require various forms of sample preparation depending on the analyte of interest, which can include plasma separation and cellular lysis. Specifically, assays that require the release of intraerythrocytic analytes (e.g., detection of malaria antigens, dehydrogenases, and hemoglobin) require the rupture of red blood cells prior to analysis. Associated handling steps and additional fluid manipulation complicates the user-experience by adding time, potential for contamination error, and reagent waste. In this work, we demonstrate an in situ chemical hemolysis treatment coupled with a paper-based device for the quantification of liberated hemoglobin without using a hemolytic buffer. In contrast to traditional hemolytic methods that use a buffered solution of saponin, a surfactant, we dried saponin within our device to lyse red blood cells without diluting the sample. The optimal treatment condition for hemolysis of blood samples with hematocrit values ranging from 20–50% was 10.6 μg saponin per cm2. Establishing a relationship between saponin and zone area potentially allows this in situ hemolysis treatment to be translated to other paper-based devices with different geometries. For samples with hematocrit values below 40%, we achieved quantitative hemolysis. Samples with higher hematocrits (e.g., 40–50%) experienced a lesser extent of hemolysis (80–85%), which we attribute to the increased number of red blood cells present in samples with elevated hematocrits. The in situ chemical hemolysis treatment described here could potentially be integrated with a multiplexed paper-based microfluidic device to permit multiple sample preparation techniques on a single sample of blood without additional off-chip user steps.
- This article is part of the themed collection: Bioanalytical sensors for real world applications


 Please wait while we load your content...
Please wait while we load your content...