A microfluidic organotypic device for culture of mammalian intestines ex vivo†
Abstract
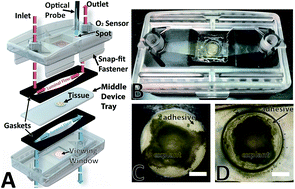
The physiological characteristics of the gastrointestinal (GI) tract are diverse and include rapid rates of epithelial turnover, complex nervous and immune systems, a thick mucus layer, and a large microbial population. Most GI models in vitro rely upon cell lines or organoids and consequently lack the diversity of cells and microorganisms present in vivo. In vivo studies retain function and cellular diversity but are more difficult to control. Microfluidic tissue-on-a-chip devices provide powerful alternatives for modeling physiological systems. Such devices show promise for use in GI research; however, most models use non-physiologic culture environments with higher than in vivo oxygen levels and insufficient gut microbiota. Our goal is to create a bridge between in vitro and in vivo using microfluidic devices by incorporating ex vivo tissue explants in physiologically relevant environments. Here, we report a microfluidic organotypic device (MOD) that enables media flow with differential oxygen concentrations across luminal and muscular surfaces of gut tissue ex vivo. Tissue was shown to be viable for 72 h and lowering oxygen concentration to a more physiologic level impacted bacterial populations.


 Please wait while we load your content...
Please wait while we load your content...