The reduction performance of double bonds regulated by the competition of push–pull electron groups to realize the colorimetric and fluorescence recognition of hypochlorous acid†
Abstract
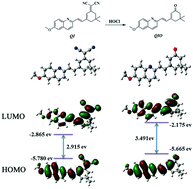
Based on its reducibility, the double bond can act as a reaction site for hypochlorous acid (HOCl), which had been demonstrated by a great deal of work. Nevertheless, the reactivity is influenced by the adjacent chemical environment. Therefore, in this work, we constructed a probe (QI) by methoxy-substituted quinoline conjugating dicyanoisoflurone, in which dicyano and pyridine N act as electron-withdrawing groups and the methoxy acts as an electron-donating group, to regulate their adjacent C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C reactivity. The “push–pull” electron effect between the methoxy group and the pyridine N led to the C
C reactivity. The “push–pull” electron effect between the methoxy group and the pyridine N led to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond being passivated. On the other hand, another C
C bond being passivated. On the other hand, another C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond was activated by the strong electron-pulling effect of the dicyano group. Thus, the previously weak intramolecular charge transfer became stronger after the dicyano adjacent to the C
C bond was activated by the strong electron-pulling effect of the dicyano group. Thus, the previously weak intramolecular charge transfer became stronger after the dicyano adjacent to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C was oxidized by HOCl, and showed a strong emission shifted from 570 to 520 nm along with a color change. The reaction mechanism was verified by mass spectrometry, NMR and theoretical calculation, and further bioimaging demonstrated the practical application of the probe.
C was oxidized by HOCl, and showed a strong emission shifted from 570 to 520 nm along with a color change. The reaction mechanism was verified by mass spectrometry, NMR and theoretical calculation, and further bioimaging demonstrated the practical application of the probe.


 Please wait while we load your content...
Please wait while we load your content...