A simplified protein purification method through nickel cleavage of the recombinant protein from the Escherichia coli cell surface†
Abstract
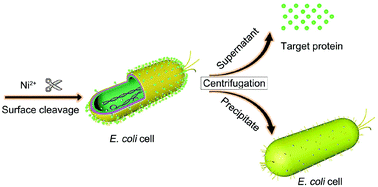
To simplify the protein purification process, we developed a novel one-step purification method in which the recombinant protein can be cleaved directly from the Escherichia coli cell surface. This method involves fusion of the target protein to the C-terminus of a LOS tag comprising a surface anchor protein (Lpp-OmpA) and a sequence-specific nickel-assisted cleavage (SNAC)-tag. The LOS tag facilitates the anchoring of the target protein to the outer membrane of E. coli cells and its separation from the cell membrane through Ni2+ cleavage. Intact, biologically active protein with a purity of 95% and a yield of approximately 100 mg per liter of culture can be readily obtained through Ni2+ cleavage in resuspension solution followed by centrifugation. In this study, a practical and promising protein purification method has been established with minimal labor and cost, as no cell disruption and chromatographic separation are required downstream.
- This article is part of the themed collection: Celebrating 100 years of Chemistry at Nanjing University


 Please wait while we load your content...
Please wait while we load your content...