Picoliter enzyme reactor on a nanofluidic device exceeding the bulk reaction rate†
Abstract
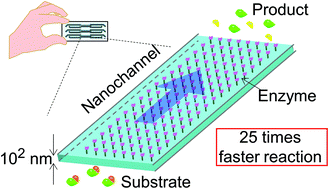
Single-cell analyses have recently become important to understand cell heterogeneity, the mechanism of cell function, and diseases. In contrast to single-cell analyses that target nucleic acids, single-cell protein analyses still pose challenges. We have proposed a general concept of integration and extended this concept to the 10–1000 nm scale with femtoliter–picoliter volumes which are smaller than the volume of a single cell exploring ultimate analytical performances (e.g. single-cell target proteomics). However, single-cell shotgun proteomics, which is used to analyze even unknown proteins, is still challenging because there is no digestion column with picoliter volume. The issues were long reaction time (overnight) and much larger reaction volume (microliter) in the conventional bulk method. In this study, an ultra-fast picoliter enzyme reactor using a nanochannel was developed. A device with a channel depth of 300 nm and a volume of 32.4 pL was fabricated. To prevent the self-digestion of trypsin (enzyme), the picoliter enzyme reactor was prepared by immobilizing trypsinogen which was activated to trypsin by enterokinase. The enzyme density obtained by the trypsinogen immobilization process was 2.5 times higher than that obtained by the conventional trypsin immobilization process. Furthermore, the apparent enzyme concentration was 36 times higher due to an extremely high surface-to-volume ratio of the nanochannel, compared to the limit concentration in the bulk. Finally, the enzyme reaction in the picoliter enzyme reactor was accelerated 25 times compared to that in the bulk. Using the picoliter enzyme reactor, protein solution with picoliter volume will be digested without self-digestion and artificial modification, which will greatly contribute to single-cell shotgun proteomics.


 Please wait while we load your content...
Please wait while we load your content...