A new chromogenic and fluorescent chemosensor based on a naphthol–bisthiazolopyridine hybrid: a fast response and selective detection of multiple targets, silver, cyanide, sulfide, and hydrogen sulfide ions and gaseous H2S†
Abstract
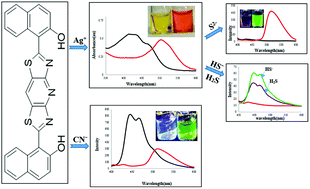
A novel chemosensor 1 based on a naphthol–bisthiazolopyridine hybrid was synthesized and characterized. Among the various screened cations and anions, chemosensor 1 selectively recognized Ag+ and CN− ions by relying on the color change and distinct absorption and emission changes. Moreover, the probe 1·Ag+ was well operable for the selective monitoring of S2−, HS− and H2S as evidenced by the different color and optical changes. The selective detection of all the anions was simply based on the breakage of the intramolecular hydrogen bonding of 1, followed by the protonation or deprotonation mechanism. In general, chemosensor 1 is a promising indicator in terms of its ease-of-use, selectivity, sensitivity, and rapid response (<1 s). Moreover, the visual detection and concentration determination of these analytes by solution or solid kits are the advantages for the practical applications of this sensor.


 Please wait while we load your content...
Please wait while we load your content...