Clinical blood sampling for oxylipin analysis – effect of storage and pneumatic tube transport of blood on free and total oxylipin profile in human plasma and serum†
Abstract
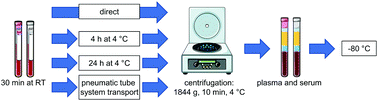
Quantitative analysis of oxylipins in blood samples is of increasing interest in clinical studies. However, storage after sampling and transport of blood might induce artificial changes in the apparent oxylipin profile due to ex vivo formation/degradation by autoxidation or enzymatic activity. In the present study we investigated the stability of free (i.e. non-esterified) and total oxylipins in EDTA-plasma and serum generated under clinical conditions assessing delays in sample processing and automated transportation: Free cytochrome P450 monooxygenase and 5-lipoxygenase (LOX) formed oxylipins as well as autoxidation products were marginally affected by storage of whole blood up to 4 h at 4 °C, while total (i.e. the sum of free and esterified) levels of these oxylipins were stable up to 24 h and following transport. Cyclooxygenase (COX) products (TxB2, 12-HHT) and 12-LOX derived hydroxy-fatty acids were prone to storage and transport induced changes due to platelet activation. Total oxylipin patterns were generally more stable than the concentration of free oxylipins. In serum, coagulation induced higher levels of COX and 12-LOX products showing a high inter-individual variability. Overall, our results indicate that total EDTA-plasma oxylipins are the most stable blood oxylipin marker for clinical samples. Here, storage of blood before further processing is acceptable for a period up to 24 hours at 4 °C. However, levels of platelet derived oxylipins should be interpreted with caution regarding potential ex vivo formation.


 Please wait while we load your content...
Please wait while we load your content...