Glycosylated naphthalimides and naphthalimide Tröger's bases as fluorescent aggregation probes for Con A†
Abstract
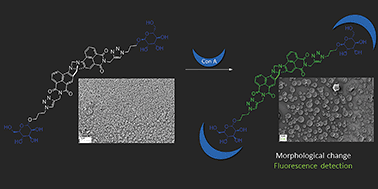
Herein we report the synthesis of fluorescent, glycosylated 4-amino-1,8-naphthalimide (Nap) 1, and the related 1,8-naphthalimides Tröger's bases (TBNap) 2 and 3, from 1,8-naphthalic anhydride precursors, the α-mannosides being introduced through the use of CuAAC mediated ‘click’ chemistry. We investigate the photophysical properties of these probes in buffered solution and demonstrate their ability to function as fluorescent probes for Concanavalin A (Con A) lectin. We show that both the Nap and TBNap structures self-assemble in solution. The formation of the resulting supramolecular structures is driven by head-to-tail π–π stacking and extended hydrogen bonding interactions of the Nap and the triazole moieties. These interactions give rise to spherical nano-structures (ca. 260 nm and 100 nm, for 1 and 3, respectively), which interact with the Con-A protein, the interaction being probed by using both luminescent and Scanning Electron Microscopy imaging as well as dynamic light scattering measurements. Finally, we show that these supramolecular assembles can be used as luminescent imaging agents, through confocal fluorescence imaging of HeLa cells of the per-acetylated version 2.


 Please wait while we load your content...
Please wait while we load your content...