A new indium selenide phase: controllable synthesis, phase transformation and photoluminescence properties†
Abstract
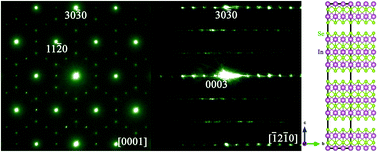
A new indium selenide phase, close to the composition of In2.45Se4, is controllably synthesized by an ethylenediaminetetraacetic acid (EDTA) mediated solvothermal method. Through detailed structural and chemical characterization using transmission electron microscopy and synchrotron powder X-ray diffraction, it is determined that In2.45Se4 crystallizes in a rhombohedral structure with lattice parameters of a = 6.9632(1) Å and c = 38.1612(1) Å. Systematic investigations show that the synthesis parameters, including the reaction temperature, EDTA/InCl3 molar ratio, solvent composition, and reaction duration, play crucial roles in the formation of this new indium selenide phase. Naturally, such a new-phase In2.45Se4 easily forms flowerlike nano/micro-structures, while In2.45Se4 hexagonal nanoplates can also be selectively synthesized by tuning the precursor concentration. In situ high-temperature synchrotron powder X-ray diffraction analysis demonstrates that In2.45Se4, with a hexagonal nanoplate morphology, is stable up to 400 °C, while a phase transformation from In2.45Se4 to γ-In2Se3 takes place when the annealing temperature reaches 500 °C. The photoluminescence property measurements show that the In2.45Se4 hexagonal nanoplates have a near infrared emission at ∼815 nm, indicating their potential applications in optoelectronics.


 Please wait while we load your content...
Please wait while we load your content...