Dithienosilole-co-5-fluoro-2,1,3-benzothiadiazole-containing regioisomeric polymers for organic field-effect transistors†
Abstract
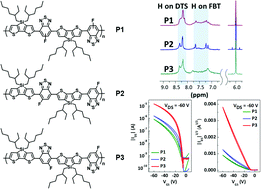
Three regioisomeric polymers (P1, P2, and P3) comprising dithienosilole and asymmetric 5-fluoro-2,1,3-benzothiadiazole moieties are synthesized and characterized. Although all the polymers exhibit similar energy levels, they show different molecular orientation, crystallinity, and charge-carrier transporting characteristics. A superior hole mobility (0.021 cm2 V−1 s−1) is demonstrated for an organic field-effect transistor (OFET) based on P3 with the geometrical same direction of the F atoms, which is much higher than the regiorandom (P1) and regioregular (P2) isomers. Our study reveals that the specific regio-orientation of the asymmetric units rather than the regioregularity in the backbone plays a crucial role in determining the polymer nature, bulk morphology, and OFET performance.


 Please wait while we load your content...
Please wait while we load your content...