Slow relaxation of magnetization in unprecedented Cu–Ln-Rad hetero-tri-spin chains constructed from multidentate nitronyl nitroxide†
Abstract
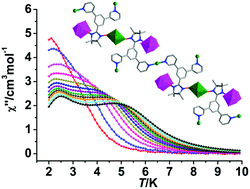
Reactions of the radical 3,5-bPy-Ph-Nit (2-[3,5-bis(3-pyridyl)-phenyl]-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide) with Ln(hfac)3 and Cu(hfac)2 afforded four unprecedented radical-3d–4f chains with formula {[Ln(hfac)3][Cu(hfac)2]2(3,5-bPy-Ph-Nit)(H2O)}n·nC7H16 (Ln = Gd, 1; Tb, 2; Dy, 3; Ho, 4; hfac = hexafluoroacetylacetonate). In complexes 1–4, each 3,5-bPy-Ph-Nit radical serves as one μ4-bridge to bind one LnIII ion and three CuII ions via its two aminoxyl units and two pyridine-N atoms, giving rise to unique Cu–Ln-radical one-dimensional chains with a rare [Ln-NIT-Cu-NIT-Ln] structural motif. Magnetic investigation shows that ferromagnetic interactions are found between the aminoxyl unit and Cu(II) or Ln(III) ions. Interestingly, a Dy derivative displays two-step relaxations of magnetization, while a Tb complex exhibits field-induced slow magnetic relaxation, originating from the [Ln-NIT-Cu-NIT-Ln] ferromagnetic structural units.


 Please wait while we load your content...
Please wait while we load your content...