Theoretical investigations of the realization of sky-blue to blue TADF materials via CH/N and H/CN substitution at the diphenylsulphone acceptor†
Abstract
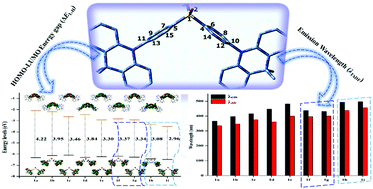
A series of derivatives based on 10,10-dimethyl-5,10-dihydro-pyrido[4,3-b][1,6]naphthyridine–diphenylsulphone (DMDHNP-DPS) named 1a was designed with CH/N and H/CN substitution at the DPS acceptor unit to obtain blue thermally activated delayed fluorescence (TADF) materials. The parent molecule 1a was chosen from our previous report after CH/N substitution at the DMAC donor fragment. The highest occupied molecular orbitals (HOMOs) and the lowest unoccupied molecular orbitals (LUMOs) were largely distributed over the DMDHNP donor and DPS acceptor units, respectively, resulting in a slight overlap between the HOMO–LUMO and hence a smaller singlet–triplet energy gap (ΔEST). Steric hindrance caused a large dihedral angle (≈82°–89°) between the plane of the electron-donating DMDHNP unit and the electron-accepting DPS unit in the substituted derivatives. Calculated results indicated that the ΔEST values of H/CN substituted derivatives were smaller than those of the corresponding CH/N derivatives which were favorable for the reverse intersystem crossing (RISC) process from the lowest excited triplet states (T1) to the lowest excited singlet (S1) states and ultimately to the ground state (S0) causing delayed emission. The emission wavelengths (λem) of all the designed molecules were found to be in the range of 397–497 nm. The incorporation of the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) atom or the –CN group at the ortho and meta positions of DPA reduced the transition energies from LUMO → HOMO in the S1 states, resulting in a red-shift. Moreover, the λem values displayed a more substantial bathochromic-shift as the number of –N
atom or the –CN group at the ortho and meta positions of DPA reduced the transition energies from LUMO → HOMO in the S1 states, resulting in a red-shift. Moreover, the λem values displayed a more substantial bathochromic-shift as the number of –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) atoms or –CN groups increased. The two of the designed molecules (1h and 1i) showed sky-blue emission (494 nm and 497 nm), and the four of the investigated compounds (1c, 1d, 1f, and 1g) displayed blue emission (416 nm, 447 nm, 437 nm, and 432 nm, respectively) indicating that these investigated derivatives were efficient sky-blue to blue TADF candidates. Among all the investigated derivatives, the smaller ΔEST values for the designed systems 1f (0.03 eV) and 1g (0.02 eV) and appropriate λem values of 437 nm and 432 nm make them excellent candidates for blue TADF materials. Our theoretical investigation might offer hints for the construction of efficient blue TADF-based organic light emitting diodes (OLEDs) in the future.
atoms or –CN groups increased. The two of the designed molecules (1h and 1i) showed sky-blue emission (494 nm and 497 nm), and the four of the investigated compounds (1c, 1d, 1f, and 1g) displayed blue emission (416 nm, 447 nm, 437 nm, and 432 nm, respectively) indicating that these investigated derivatives were efficient sky-blue to blue TADF candidates. Among all the investigated derivatives, the smaller ΔEST values for the designed systems 1f (0.03 eV) and 1g (0.02 eV) and appropriate λem values of 437 nm and 432 nm make them excellent candidates for blue TADF materials. Our theoretical investigation might offer hints for the construction of efficient blue TADF-based organic light emitting diodes (OLEDs) in the future.


 Please wait while we load your content...
Please wait while we load your content...