Synergistic effects of hydrogen bonds and the hybridized excited state observed for high-efficiency, deep-blue fluorescent emitters with narrow emission in OLED applications†
Abstract
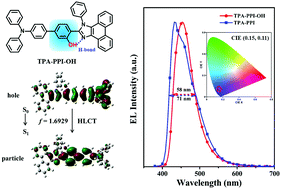
Efficient deep-blue fluorescence and high color purity (narrow emission) are highly desired characteristics for organic light-emitting diodes (OLEDs). Herein, we report on hydrogen-bond (H-bond)-induced narrow emission based on a donor–acceptor-type molecule TPA-PPI-OH with a hydroxyl (–OH) substituent. Nuclear magnetic resonance (NMR) spectroscopy and single X-ray crystal data indicated the existence of intra- and intermolecular H-bonds interactions in TPA-PPI-OH. These interactions proved beneficial to suppress the structural vibrations and thereby caused a narrower full-width at half-maximum (FWHM) PL emission of TPA-PPI-OH compared to its hydroxy-free counterpart TPA-PPI (57 nm vs. 63 nm in film). The photophysical properties revealed that the lowest excited state (S1) of TPA-PPI-OH is a hybridized local and charge-transfer excited state; thus, TPA-PPI-OH could show high fluorescence efficiencies in various solvents (50% even in acetonitrile). The non-doped deep-blue device based on TPA-PPI-OH exhibited a maximum EQE of 7.37% with a small efficiency roll-off (7.37% @ 100 cd m−2; 5.48% @ 1000 cd m−2) and narrow FWHM of 58 nm (71 nm for TPA-PPI).


 Please wait while we load your content...
Please wait while we load your content...