Enantiopure versus racemic naphthalene diimide-based n-type organic semiconductors: effect on charge transport†
Abstract
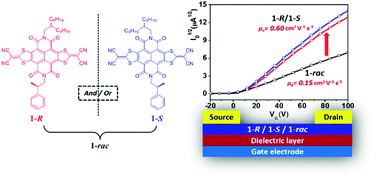
Chiral alkyl chains are widely utilized to ensure the solubility of solution-processed organic semiconductors (OSCs), while intrinsic defects due to the mixture of several stereoisomers in one material are frequently neglected. Herein, through introducing an optically pure pendant into the molecular backbone of a core-expanded naphthalene diimide (NDI-DTYM2), enantiopure semiconductor materials (1-R/1-S) and the corresponding racemate (1-rac) were designed and synthesized to investigate the impact of enantiopure and racemic OSCs on charge transport in organic field-effect transistors (OFETs). Surprisingly, a 2–4 times increase in electron mobility (from 0.15 cm2 V−1 s−1 to 0.6 cm2 V−1 s−1 for as-cast devices and from 0.42 cm2 V−1 s−1 to 0.98 cm2 V−1 s−1 for thermally-annealed devices) was discovered in solution-processed OFETs simply by switching the active layer material from racemic to the enantiopure form. Grazing-incidence wide-angle X-ray scattering (GIWAXS), atomic force microscopy (AFM) and were utilized to investigate the crystallization, molecular micro-organization and film morphology of 1-R, 1-S and 1-rac, demonstrating that the lower mobility of racemic material (1-rac) might be attributed to interfacial defects among different crystalline domains as well as subtle changes in molecular packing.


 Please wait while we load your content...
Please wait while we load your content...