Effects of cobalt substitution on ZnO surface reactivity and electronic structure
Abstract
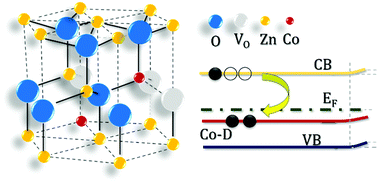
We have performed scanning probe microscopy investigations of ZnO and Co-substituted ZnO under dark/UV conditions as well as in air and an ultra-high vacuum environment to shine a light on the change in electronic structure and surface reactivity as a consequence of Zn substitution with Co. We have achieved two major results: first, Co substituting Zn atoms significantly downward shifts by about 400 meV the Fermi level, which is close to the conduction band in the as-grown n-type ZnO. Second, a thoroughly novel result, Co substitution strongly reduces the absorption of negative oxygen species (NOS) at the ZnO surface. These two experimental findings are fully explained by a phenomenological model assuming the formation of Co-defect (Co-D) complexes that induce the appearance of an unoccupied impurity band in the ZnO energy gap. NOS play a central role in both the operating principles of UV photodetectors and applications in nanomedicine. Thus, the inhibiting effect of Co-D complexes on NOS formation has many applicative implications since it suggests that defect-engineering procedures might be devised for realizing nano-patterned Co-doped ZnO surfaces with regions showing different surface properties.


 Please wait while we load your content...
Please wait while we load your content...