Incorporation of short, charged peptide tags affects the temperature responsiveness of positively-charged elastin-like polypeptides†
Abstract
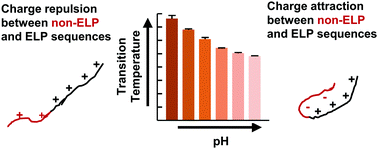
Elastin-like polypeptides (ELPs) are recombinant protein domains exhibiting lower critical solution temperature (LCST) behavior. This LCST behavior is controlled not only by intrinsic factors including amino acid composition and polypeptide chain length but also by non-ELP fusion domains. Here, we report that the presence of a composite non-ELP sequence that includes both His and T7 tags or a short Ser-Lys-Gly-Pro-Gly (SKGPG) sequence can dramatically change the LCST behavior of a positively-charged ELP domain. Both the His and T7 tags have been widely used in recombinant protein design to enable affinity chromatography and serve as epitopes for protein detection. The SKGPG sequence has been used to improve the expression of ELPs. Both the composite tag and the SKGPG sequence are <15% of the total length of the ELP fusion proteins. Despite the small size of the composite tag, its incorporation imparted pH-sensitive LCST behavior to the positively-charged ELP fusion protein. This pH sensitivity was not observed with the incorporation of the SKGPG sequence. The pH sensitivity results from both electrostatic and hydrophobic interactions between the composite tag and the positively-charged ELP domain. The hydrophobicity of the composite tag also alters the ELP interaction with Hofmeister salts by changing the overall hydrophobicity of the fusion protein. Our results suggest that incorporation of short tag sequences should be considered when designing temperature-responsive ELPs and provide insights into utilizing both electrostatic and hydrophobic interactions to design temperature-responsive recombinant proteins as well as synthetic polymers.


 Please wait while we load your content...
Please wait while we load your content...