Gold nanoparticle–protein conjugate dually-responsive to pH and temperature for modulation of enzyme activity†
Abstract
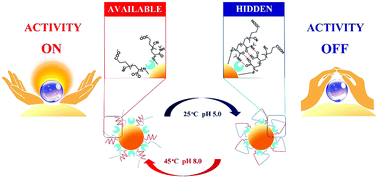
Regulation of the activity and stability of enzymes is important for their biological and biomedical applications. In this regard, considerable attention has been paid to the regulation of enzyme activity by controlling steric effects in protein–thermoresponsive polymer conjugates via temperature change. However, it is difficult to accomplish on/off regulation of activity via steric effects alone. In this work, pH- and temperature-dually-responsive gold nanoparticles (AuNPs) conjugated to poly(N-isopropylacrylamide) (pNIPAM) and poly(methacrylic acid) (pMAA), designated AuNP–pNIPAM–pMAA, are proposed in this work for the regulation of enzyme activity with high precision. Using these particles adsorbed with inorganic pyrophosphatase (PPase) as a model enzyme (AuNP–PPase–pNIPAM–pMAA), it was shown that, by changes in pH and temperature leading to changes in “steric covering” of the protein active site via interpolymer hydrogen bonding, the activity of PPase could be closely regulated. At 25 °C and pH 5.0, the steric covering of pNIPAM and the interpolymer hydrogen bonding between pNIPAM and pMAA gave a cage-like “locking in” structure in the protein active site as the “hidden” state, with a very low specific activity of only 12 U mg−1, around 2% of its normal activity. At 45 °C and pH 8.0, the cage structure did not form due to the lack of coverage by the collapsed pNIPAM chains and the inability of the dissociated carboxyl groups in pMAA to form hydrogen bonds, leaving the protein active center fully exposed as the “available” state, and resulting in a high specific activity of 498 U mg−1. Thus the activity of the enzyme in the conjugate is almost totally suppressed at 25 °C/pH 5.0 and completely recovered at 45 °C/pH 8.0. The controllable range of activity is thus very wide, i.e., from 2 to 100%. The AuNP–PPase–pNIPAM–pMAA conjugate also has strong resistance to trypsin digestion. In addition, it was shown that after cycling three times between the “hidden” and “available” states, the conjugate retained more than 85% of its initial activity, showing that this system can be cycled and used multiple times without significant loss of activity. It is concluded that this dual-responsive gold nanoparticle–protein–polymer conjugate has great potential in applications requiring close control of protein activity under complex environmental conditions.


 Please wait while we load your content...
Please wait while we load your content...