Pt-loaded Au@CeO2 core–shell nanocatalysts for improving methanol oxidation reaction activity†
Abstract
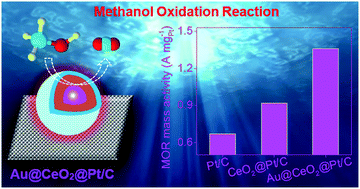
Herein, we provide a facile hydrothermal process for ultralow Pt loading (3.84 wt%) on the porous surface of an Au@CeO2 core–shell nanocatalyst (CSNC) in order to improve the electrocatalytic property of bare Pt towards the methanol oxidation reaction (MOR). The Au@CeO2@Pt CSNC demonstrated a high BET surface area (88.10 m2 g−1) and high numbers of catalytic surface active species (such as Pt0, Ce3+ and oxygen vacancies). The corresponding electrode obtained by spraying the Au@CeO2@Pt CSNC onto the microporous layer (MPL) of carbon cloth (Au@CeO2@Pt/C) showed better electrocatalytic properties, such as high electrochemical surface area (ECSA – 80 m2 g−1) and low charge transfer resistance (37 Ω) than CeO2@Pt/C (52 m2 g−1 and 106 Ω) and commercial Pt/C (44 m2 g−1 and 182 Ω). Furthermore, the positive catalytic properties of the Au@CeO2@Pt/C electrode were investigated via MOR mass activity which at 1.36 A mgPt−1, was 1.5 and 2.0 times higher than those obtained from the CeO2@Pt/C (0.92 A mgPt−1) and commercial Pt/C (0.67 A mgPt−1) electrodes, respectively. Moreover, the Au@CeO2@Pt/C electrocatalyst also had good MOR durability and high CO tolerance. The electrocatalytic enhancement of the Au@CeO2@Pt CSNC could be the result of the electronic, bifunctional and synergistic effects between the Au, CeO2 and Pt components supported on the carbon cloth. Accordingly, these advantageous effects easily removed the adsorbed CO intermediate as the main poisoner on the surface of the Pt catalyst, and thereby significantly improved the overall MOR activity.


 Please wait while we load your content...
Please wait while we load your content...