In search of the most active MN4 catalyst for the oxygen reduction reaction. The case of perfluorinated Fe phthalocyanine†
Abstract
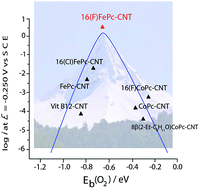
Iron macrocyclic complexes (MN4) are promising catalysts for replacing platinum (the industrial standard) in electrocatalysis. In particular, FeN4 complexes have shown lower overpotential than Pt for the oxygen reduction reaction (ORR) in alkaline media. To predict the electrochemical activity of metal electrodes and molecular catalysts towards the ORR, reactivity descriptors with typical volcano correlation have been demonstrated. The most important are M–O2 binding energy and M(n)+/M(n−1)+ redox potentials for the complexes. We studied a new Fe complex, which possesses powerful electron-withdrawing fluorine residues at the periphery of the phthalocyanine ring. Fe hexadecafluorophthalocyanine (16(F)FePc) was characterized by electron paramagnetic resonance (EPR), and X-ray photoelectron spectroscopy (XPS) in the presence and in absence of O2. Experimental and calculated O2–Fe binding energies, as well as electrochemical characterization confirms the excellent activity of this complex for the ORR placing this complex at the top of the MN4 volcano correlation.
- This article is part of the themed collection: Celebrating Latin American Talent in Chemistry


 Please wait while we load your content...
Please wait while we load your content...