Hydroxyl group modification improves the electrocatalytic ORR and OER activity of graphene supported single and bi-metal atomic catalysts (Ni, Co, and Fe)†
Abstract
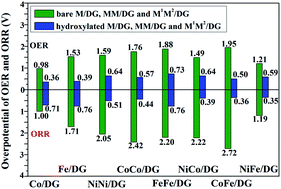
Single atomic and diatomic catalysts have been extensively studied as electrocatalysts for the oxygen evolution and oxygen reduction reactions (OER and ORR) in recent years. It was found that the interfacial chemistry between atomic catalysts and hydroxyl groups had a profound influence on the electrochemical reaction process. In this work, we carried out systematic density functional theory (DFT) studies on the electrocatalytic process of the OER and ORR under alkaline conditions over hydroxyl group modified single metal atom (M = Ni, Co, Fe) and bi-metal atoms (denoted as MM and M1M2, where M, M1 and M2 are Ni, Co, and Fe, respectively) supported on defective graphene (DG). We investigated the reaction processes of both the OER and ORR on bare and hydroxyl group modified catalysts, and the results indicated that the hydroxylated single and bi-metal (HO-M/DG, M = Co, Fe; (HO)2-MM/DG, M is Ni, Co, and Fe; (HO)2-M1M2/DG, where M1 and M1 are Ni, Co, and Fe) atoms have much higher catalytic activity for both the OER and ORR than the bare metal atoms, while the HO-Ni/DG cannot be an OER and ORR catalyst because the intermediates (OH*, O* and OOH*) could not be adsorbed stably on hydroxylated Ni/DG under the reaction conditions. With the hydroxyl group modification, the single metal atomic catalysts (M = Co and Fe) exhibited much improved activity for the OER process, while the hydroxyl group modified bi-metal atomic catalysts exhibited much improved activity toward the ORR. The reason for the promoting effect on catalytic activities of hydroxylated atomic catalysts for the ORR and OER is discussed. We hope this work provides a new perspective on catalyst design for the OER and ORR processes.


 Please wait while we load your content...
Please wait while we load your content...