Theoretical search for novel Au or Ag bimetallic alloys capable of transforming CO2 into hydrocarbons†
Abstract
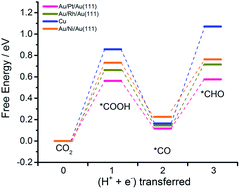
Designing efficient catalysts with a high selectivity toward hydrocarbons at a relatively low overpotential is of great significance for the application of the CO2 electrochemical reduction reaction (CO2RR). In consideration of the advantages of Au or Ag pure metal with high faradaic efficiencies towards CO and a weaker competing hydrogen evolution reaction (HER) compared to Cu, this work employed density functional theory (DFT) calculations to explore whether the modification of Au or Ag pure metal could generate hydrocarbons rather than CO, as well as retain the two advantages above. Herein, 28 kinds of bimetallic alloys were investigated with models denoted as A/S(111) and A/S/A(111), where A is Au or Ag and S is a transition metal (Au, Ag, Pt, Pd, Ir, Rh, or Ni). Both the thermodynamic and kinetic aspects of the key elementary steps towards hydrocarbons are evaluated to reveal the catalytic performance of these alloys. The results indicated that Au/Pt/Au(111), Au/Rh/Au(111) and Au/Ni/Au(111) are the most promising catalyst candidates, being able to produce hydrocarbons with high faradaic efficiency at a much lower potential.


 Please wait while we load your content...
Please wait while we load your content...