A Co–Mo2N composite on a nitrogen-doped carbon matrix with hydrogen evolution activity comparable to that of Pt/C in alkaline media†
Abstract
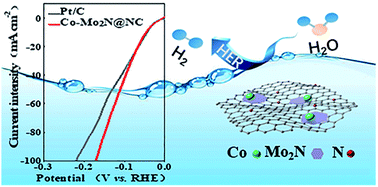
Intrinsic activity and electrical conductivity play key roles in the hydrogen evolution reaction (HER). We herein design and synthesise nanocomposites of metallic cobalt and molybdenum nitride on a nitrogen-doped carbon matrix (Co–Mo2N@NC). The electrocatalyst exhibits extraordinary HER performance with an overpotential of only 47 mV at a current density of 10 mA cm−2 and Tafel slope of 43 mV dec−1 in a 1 M KOH solution, which is comparable to that of commercial 20 wt% Pt/C (43 mV at 10 mA cm−2). At high current density it even exhibits better performance than Pt/C (170 mV vs. 220 mV at 100 mA cm−2). The excellent activity stems from the roles of Co nanoparticles in improving electrical conductivity, optimizing the electronic structure and thus speeding up hydrogen desorption from Mo2N, and of the in situ formed Co(OH)2 in boosting water dissociation. Therefore, this work provides a low-cost potential electrocatalyst to replace Pt/C.


 Please wait while we load your content...
Please wait while we load your content...