Novel carbon and sulfur-tolerant anode material FeNi3@PrBa(Fe,Ni)1.9Mo0.1O5+δ for intermediate temperature solid oxide fuel cells†
Abstract
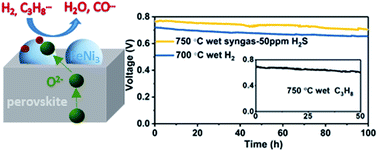
Suppression of carbon deposition and sulfur poisoning without sacrificing electrochemical performance is crucial for operating solid oxide fuel cells (SOFCs), especially at intermediate temperatures (IT). In this work, nickel-doped A-site deficient perovskite oxides (PrBa)0.95Fe1.9−xNixMo0.1O6−δ (PBFMNix, x = 0, 0.1, 0.2, 0.3, 0.4) were synthesized and investigated as potential anodes for IT-SOFCs. With increased amounts of Ni2+, the ratios of Fe2+/Fe3+ and Mo5+/Mo6+ in as-prepared PBFMNix continuously decreased under a charge-compensating mechanism, which simultaneously depressed the formation of the impurity phase (BaMoO4). Interestingly, the substitution of Ni2+ benefits the reduction of Fe3+ to Fe2+ and Mo6+ to Mo5+, and small portions of Fe and Ni elements are exsolved from the parent oxides, forming FeNi3 alloy nano-particles that greatly accelerate the chemical adsorption and surface reaction kinetics of H2, and thus improve the electrochemical performances of oxide-based anodes. Transformation of the electrical conduction from p-type to n-type after reduction was also observed. A very small polarization resistance of 0.028 Ω cm2 at 750 °C was achieved for the cell with the PBFMNi0.3 anode. Importantly, fueled with syngas with 50 ppm H2S, the maximum power density of a button cell based on the PBFMNi0.3 anode and an Sm0.2Ce0.8O1.9 (SDC) electrolyte-supporting configuration can reach 498 mW cm−2 at 750 °C, and long-term stability over 100 hours can be demonstrated with negligible performance decay. All these results indicate that PBFMNi0.3 is a promising high-performance anode material with good coking resistance and sulfur tolerance in intermediate temperatures.


 Please wait while we load your content...
Please wait while we load your content...