High-concentration ether-based electrolyte boosts the electrochemical performance of SnS2–reduced graphene oxide for K-ion batteries†
Abstract
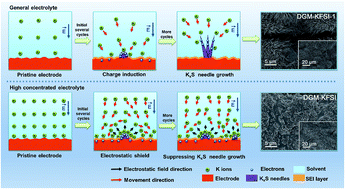
To meet the urgent demand for energy storage systems, K-ion batteries (KIBs), with low cost and comparable electrochemical performance, have become one of the most promising alternatives to Li-ion batteries. In this study, nanocrystalline SnS2 anchored to reduced graphene oxide (SnS2–RGO) is investigated using ether-based electrolytes. A reversible specific capacity of 436 mA h g−1 at 100 mA g−1 after 50 cycles was obtained, as well as the reversible specific capacity of 311 mA h g−1 at 500 mA g−1 after 150 cycles, which are much better than those using ester-based electrolytes. Interestingly, it was found that high-concentration ether-based electrolytes can remarkably suppress the growth of KxS needles, which is probably due to the electrostatic shielding effect induced by the high concentration of K ions. This discovery should be important for K-ion storage and could shed light on the design of better KIBs from the perspective of electrolytes. Furthermore, we also utilized ex situ XRD patterns to reveal the electrochemical reaction process and reaction intermediate products of SnS2–RGO with high-concentration ether-based electrolytes.


 Please wait while we load your content...
Please wait while we load your content...