Efficient piezo-catalytic hydrogen peroxide production from water and oxygen over graphitic carbon nitride†
Abstract
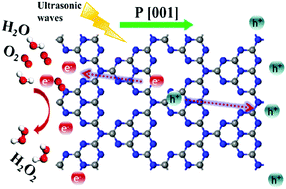
Design of a green and sustainable approach for H2O2 synthesis is a meaningful subject. Herein, we propose a piezo-catalytic method for H2O2 production by utilizing a metal-free g-C3N4 catalyst, which has high activity for generating H2O2 only from H2O and O2 under ultrasonic vibration by means of converting mechanical energy to chemical energy. Without any organics, the ultrasonic-driven generation rate of H2O2 reached 34 μmol h−1. KMnO4 reduction experiments confirmed the generation of polarized electrons for g-C3N4 under ultra-sonication, while Au deposition on the edge of g-C3N4 after piezo-catalytic reduction of HAuCl4 indicated that the ultrasonic-derived piezoelectric polarization over g-C3N4 was along the g-C3N4 plane. The construction of C or N vacancies could change the piezoelectric response of g-C3N4, which was confirmed by Piezoresponse Force Microscopy (PFM), and the result showed that the g-C3N4 containing C or N vacancies exhibited a weaker piezoelectric effect and poorer ultrasonic-assisted catalytic performance than pristine g-C3N4 due to the impairment of the crystal structure. Our work reports the piezo-catalytic effect of g-C3N4 and it may help provide a green and sustainable process of generating H2O2 directly from water and oxygen.


 Please wait while we load your content...
Please wait while we load your content...