Theoretical insights into nitrogen fixation on Ti2C and Ti2CO2 in a lithium–nitrogen battery†
Abstract
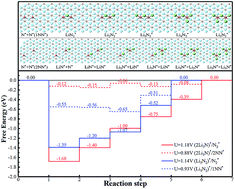
Recently, the first lithium–nitrogen (Li–N2) battery was designed, achieving a reversible electrochemical reaction between N2 and Li3N under ambient conditions (Ma et al., Chem, 2017, 2, 525–532). However, the performance of a Li–N2 battery is limited by the weak interaction between the carbon cloth cathode substrate and N2. In the present work, we theoretically evaluate a Ti2C monolayer as a potential cathode substrate to improve the performance of an Li–N2 battery. Our results reveal that N2 interacts strongly with the bare Ti2C substrate with low adsorption energy and a lot of electron transfer, and the existence of Li atoms facilitates N2 dissociation. The estimated discharge and charge overpotentials of this novel electrochemical system are less than 0.55 V. With regard to the typical surface termination, the oxygen (O) termination weakens the N2 fixation, hinders the dissociation and increases the overpotential of the electrochemical reaction. According to the present theoretical calculations, Ti2CO2 will be lithiated to Ti2CO2Li2 in the initial discharge state, and the lithiated Ti2CO2 substrate can decrease discharge and charge overpotentials in comparison to a bare Ti2CO2 substrate. Our work is the first theoretical report on the mechanism of an Li–N2 battery on a Ti2C cathode substrate, which provides a feasible strategy for efficient N2 fixation and reduction.


 Please wait while we load your content...
Please wait while we load your content...