Catalyst-free and efficient fabrication of highly crystalline fluorinated covalent organic frameworks for selective guest adsorption†
Abstract
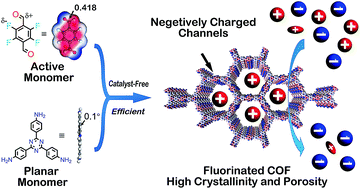
Conventional synthesis methods for imine-linked covalent organic frameworks (COFs) usually require long reaction times, tedious synthetic procedures, and indispensable catalysts, restraining the further implementation of COFs. To tackle these obstacles, we report the first catalyst-free and highly efficient fabrication method for two imine-linked COFs by virtue of rationally designed building blocks. The compound 2,3,5,6-tetrafluoroterephthalaldehyde (TFTA) was shown to be an active aldehyde monomer for accelerating the reaction and improving the crystallinity as well as the porosity. Remarkably, highly crystalline fluorinated SCF-FCOF-1 with a surface area exceeding 1000 m2 g−1 was formed within 1 hour, and COFs with surface areas as high as 2056 m2 g−1 were obtained after 3 days, representing the highest BET surface area for a fluorinated porous organic material. Moreover, the resultant fluorinated SCF-FCOF-1 exhibited excellent selective sorption performance and good recyclability toward positively charged organic molecules, presumably due to the high surface area, crystallinity, hydrophobicity and electrostatic attraction between the fluorine atoms and guest molecules.


 Please wait while we load your content...
Please wait while we load your content...