Neutral-pH overall water splitting catalyzed efficiently by a hollow and porous structured ternary nickel sulfoselenide electrocatalyst†
Abstract
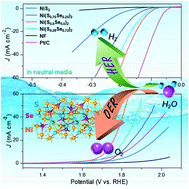
Development of nonprecious, efficient, and stable electrocatalysts for overall water splitting under mild conditions is crucial but still a huge challenge for the renewable energy techniques. Herein, a facile hydrothermal and then selenization strategy is proposed to synthesize hierarchical nickel sulfoselenide (NiS2(1−x)Se2x) hollow/porous spheres with controllable composition for electrocatalytic water splitting in neutral media. By regulating the degree of selenization, the Se/S atomic ratio in the nickel sulfoselenides can be controlled to realize the tunable electronic structure of nickel to boost its intrinsic activity. Benefiting from their unique structural features and the anion doping effect, nickel sulfoselenides, especially with the chemical composition Ni(S0.5Se0.5)2, are discovered to exhibit high activity and stability toward both hydrogen and oxygen evolution reactions under neutral conditions. When using Ni(S0.5Se0.5)2 as a bifunctional electrode as both the anode and cathode in a neutral electrolyte, it demonstrates a durable activity for overall water splitting to drive 10 mA cm−2 at a cell voltage of merely 1.87 V, outperforming the noble metal-based Pt/C and IrO2 couple. This study not only offers a new type of promising earth-abundant electrocatalyst towards water electrolysis under benign conditions, but also highlights that anion doping engineering or composition control can be an elegant strategy to improve the electrochemical catalytic performance.


 Please wait while we load your content...
Please wait while we load your content...