Salt-enhanced photocatalytic hydrogen production from water with carbon nitride nanorod photocatalysts: cation and pH dependence†
Abstract
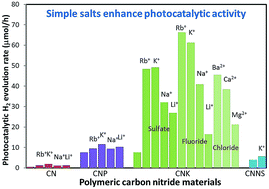
Carbon nitride polymeric semiconductors with nanorod morphology are explored as photocatalysts for efficient hydrogen evolution from water. Their composition, structure, optical properties and photocatalytic performance are found to be highly sensitive to the presence of added salts in the photocatalytic reaction solution. In potassium-incorporated carbon nitride (CNK), the photocatalytic hydrogen evolution activity is significantly enhanced with the addition of salts into the reaction solution by up to ∼9 times, depending on the salt added. Similarly, cation-dependent red-shifts in the UV-vis absorbance of CNK are observed. We find that the effect of the salts on the photocatalytic activity and optical properties of CNK is further modulated by the solution pH. The CNK nanorods form a compact layer stacking structure for certain cations, such as for K+ but not Li+. The enhancement of the photoactivity of CNK in the presence of salts is attributed to improved light harvesting ability, a dielectric screening effect, and the nanorod structure. An obvious and consistent potassium–potassium interaction is observed via EXAFS, indicating a high degree of order, likely in the form of a potassium channel or ordered layer. The strong sensitivity of CNK materials to the photocatalytic environment yields dramatic changes in structure, optical properties and photoactivity, thus providing a new experimental variable for photocatalyst material development and optimization.


 Please wait while we load your content...
Please wait while we load your content...