Integrating amino groups within conjugated microporous polymers by versatile thiol–yne coupling for light-driven hydrogen evolution†
Abstract
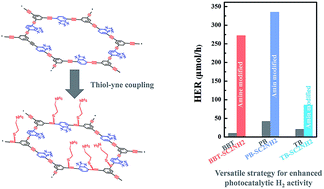
Conjugated microporous polymers (CMPs) are emerging as promising catalysts for photocatalytic hydrogen evolution, but hydrophobic surfaces and less active site exposure severely limit their efficiency. Herein, we contribute an effective and versatile strategy to functionalize CMPs with abundant amino groups via the radical thiol–yne reaction. As a result, the modified CMPs retain their light absorption ability and morphology, and the better water compatibility along with increased active site exposure may accelerate subsequent proton reduction under visible light irradiation (λ > 420 nm). The hydrogen evolution rate (HER) and apparent quantum yield (AQY) at 420 nm of modified CMPs were increased up to 27.2 times and 47.1 times in comparison to those of original CMPs. Photocatalytic H2 evolution activity evaluation of BBT-SC2CH3, BBT-SC2N(CH3)2 and SC2NHAc in which amino groups were replaced with methyl, dimethyl amino or N-acetyl groups revealed the crucial role of nitrogen, and the aliphatic chain length between sulfur and nitrogen also proved important. Therefore, this protocol provides good opportunities for designing advanced CMPs and expands their application as photocatalysts for energy conversion.


 Please wait while we load your content...
Please wait while we load your content...