Palladium structure engineering induced by electrochemical H intercalation boosts hydrogen evolution catalysis†
Abstract
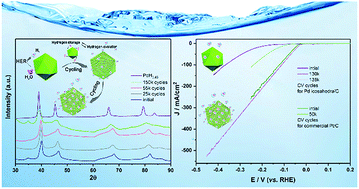
Palladium (Pd) is a versatile and active catalyst in various electrochemical applications. However, strong Pd–H bonding makes H desorption the rate-limiting step in the hydrogen evolution reaction (HER) process. Engineering material structures at the atomic level has attracted growing interest to tune the physicochemical properties and modulate the reactivity of catalysts. Here, we proposed a strategy to boost the electrocatalytic activity of Pd by electrochemical H intercalation and in situ formation of PdHx, which could consequently weaken the hydrogen adsorption on the surface of the catalyst and facilitate hydrogen generation in turn. As predicted, the Pd catalyst exhibited an unusual performance, in which repeated catalysis of hydrogen evolution resulted in continual and distinct enhancement of activity. Notably, the optimum catalytic activity was achieved after 130 000 CV cycles, in which the overpotential of 32 mV at 10 mA cm−2 was much smaller than that of initial Pd; the Pd catalyst even outperformed commercial Pt/C, indicating its extremely high durability and activity. The experimental and density functional theory (DFT) calculations revealed that the structure engineering of Pd nanocrystals induced by electrochemical H intercalation can weaken the Pd–H bonding, which boosts hydrogen evolution catalysis. Besides, the formation of PdHx by the intercalation of electrochemical H also provides an innovative stable and high-capacity hydrogen storage method.


 Please wait while we load your content...
Please wait while we load your content...