Ultrastable and efficient H2 production via membrane-free hybrid water electrolysis over a bifunctional catalyst of hierarchical Mo–Ni alloy nanoparticles†
Abstract
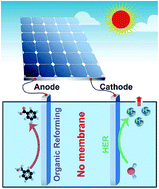
The development of an ultrastable and efficient technology for H2 production is an important step in the utilization of renewable and clean energy. Herein, we report that hybrid water electrolysis, integrating the electrochemical reforming of benzyl alcohol (BA) and the H2 evolution reaction (HER), largely accelerates the kinetics and stability of H2 production over a hierarchical bifunctional catalyst couple of Mo–Ni alloy nanoparticles in 1.0 M KOH. The high-performance of the hierarchical Mo–Ni nanoparticle catalyst for both the HER and the BA oxidation not only eliminated the need for an ion-conducting membrane to separate the cathodic and anodic reactions to achieve nearly unity faradaic efficiences for the two reactions, but also allowed the reactions to be readily driven by low cell voltage in a two-electrode configuration. Specifically, the cell voltages required to deliver the benchmark current densities of 20, 50, and 100 mA cm−2 are as low as 1.40, 1.45, and 1.53 V, respectively. The membrane-free hybrid water electrolysis technology exhibits a set of advantages including long device lifetime, ease of H2 collection, and high efficiency, thus showing significant promise for practical H2 production, especially considering the fact that the device can be readily powered by a single-cell alkaline battery or a solar cell under natural sunlight irradiation.


 Please wait while we load your content...
Please wait while we load your content...