Exploring the charge reactions in a Li–O2 system with lithium oxide cathodes and nonaqueous electrolytes†
Abstract
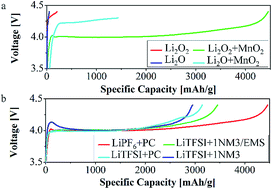
Nonaqueous lithium–oxygen batteries have attracted considerable attention due to their high energy density. Huge efforts have been made to unravel the fundamentals of Li–O2 battery chemistry. However, current Li–O2 batteries still suffer from several unresolved problems such as the instability of electrolytes and sluggish oxidation of lithium oxides during the charging process. In this work, we propose a detailed study to investigate the charge mechanism of lithium oxide materials in different electrolytes. Commercially available lithium peroxide and lithium oxide have been employed as cathodes to determine how lithium oxides (both lithium oxide and lithium peroxide) and electrolytes change during charge. The result shows that Li2O2 decomposed to lithium and oxygen; meanwhile, the electrolyte has a significant influence on Li2O2 decomposition. Furthermore, while most of the Li2O material participates in side reactions with the electrolyte, some of it is found to delithiate and crumble in structure.


 Please wait while we load your content...
Please wait while we load your content...