Mapping the frontiers of quinone stability in aqueous media: implications for organic aqueous redox flow batteries†
Abstract
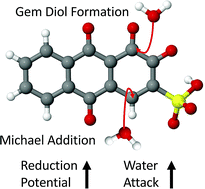
Quinone–hydroquinone pairs have been proposed as biologically-inspired, low-cost redox couples for organic electrolytes for electrical energy storage, particularly in aqueous redox flow batteries. In their oxidized form, quinones are electrophiles that can react with the nucleophilic water solvent resulting in loss of active electrolyte. Here we study two mechanisms of nucleophilic addition of water, one reversible and one irreversible, that limit quinone performance in practical flow batteries. Using a combination of density functional theory and semi-empirical calculations, we have quantified the source of the instability of quinones in water, and explored the relationships between chemical structure, electrochemical reduction potential, and decomposition or instability mechanisms. The importance of these mechanisms was further verified through experimental characterization of a family of alizarin-derived quinones. Finally, ∼140 000 prospective quinone pairs (over 1 000 000 calculations including decomposition products) were analyzed in a virtual screening using the learned design principles. Our conclusions suggest that numerous low reduction potential molecules are stable with respect to nucleophilic addition, but promising high reduction potential molecules are much rarer. This latter fact suggests the existence of a stability cliff for this family of quinone-based organic molecules, which challenges the development of all-quinone aqueous redox flow batteries.


 Please wait while we load your content...
Please wait while we load your content...