Understanding the cathode electrolyte interface formation in aqueous electrolyte by scanning electrochemical microscopy†
Abstract
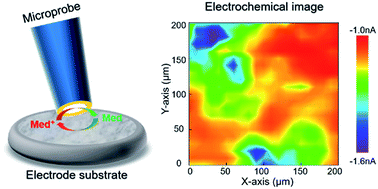
Cathode electrolyte interface (CEI) evolution in aqueous lithium-ion batteries has been studied for the first time via in situ scanning electrochemical microscopy (SECM). The results showed that a discontinuous CEI with the conductive property was formed on the LiMn2O4 cathode with the cycling process. The formation mechanism of the CEI was analyzed in combination with microscopic and spectroscopic techniques, such as TEM and XPS. The CEI with an amorphous structure, which could impede the dissolution of Mn, was formed with the help of the critical salt LiTOf. This work should be helpful in understanding the interface structure, its correlation with the cycling process, and to further improve their performances in aqueous lithium-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...