Akin solid–solid biphasic conversion of a Li–S battery achieved by coordinated carbonate electrolytes†
Abstract
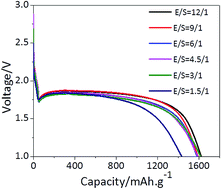
Lithium–sulfur batteries are of significant interest because their theoretical energy density exceeds that of Li-ion batteries and they can be fabricated at much lower costs. Although significant efforts have been devoted towards improving sulfur electrodes, the battery requires a “flooded” electrolyte for the dissolution–precipitation chemistry to achieve acceptable energy efficiency (the electrolyte/sulfur ratio, E/S ratio, is typically much higher than 5 : 1 μl mg−1). Herein, we report a complex electrolyte of LiTFSI salt with widely used carbonate solvents (EC and DEC) other than ether (DOL and DME) for use in Li–S batteries based on S8 as the active material. By tuning the electrolyte coordination structure, we demonstrated that the sulfur speciation pathway was fundamentally altered from the conventional dissolution–precipitation category to an akin solid–solid biphasic conversion, leading to low E/S ratios and no shuttle effect. Furthermore, the reduced reactivity of the fully coordinated solvents mitigates Li dendritic formation and related electrolyte consumption. These combined merits allow us to demonstrate that a S8/Ketjenblack electrode can achieve a capacity close to theoretical capacity (1600 mA h g−1) and high coulombic efficiency (above 99%) with a stable cycling performance at an ultralow electrolyte/sulfur ratio (1.5 : 1 μl mg−1); hence, our study defines a new pathway towards the fabrication of highly robust Li–S batteries for high-density energy storage.


 Please wait while we load your content...
Please wait while we load your content...